
Ph.D. - 1965 - Columbia University
Postdoc - 1965-66 - Harvard University
M.D. - 1975 - University of Minnesota
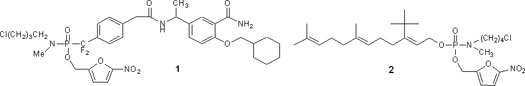
Our laboratory has a long-standing interest in the development of new drugs for the treatment of cancer. Current efforts are focused on the design, synthesis and activation mechanisms of novel prodrugs that undergo enzyme-catalyzed activation in the tumor cell to liberate a toxic phosphoramidate, phosphate or phosphonate. Several different targets are under investigation to exploit this approach. First, we have applied this novel prodrug chemistry to the design and synthesis of novel phosphotyrosine peptidomimetic prodrugs (1) that interfere with cell signaling pathways regulating cell proliferation. Cell-based assays have confirmed that the phosphotyrosine peptidomimetic prodrugs deliver the bioactive phosphate and inhibit tumor cell proliferation. We have also extended this chemistry to the synthesis of phosphatase-resistant phosphopeptidomimetics by incorporating a difluoromethylphosphonate group as a phosphate surrogate. This provides technology for the design, synthesis and intracellular delivery of long-lived phosphate-based antagonists and phosphatase inhibitors. In collaboration with the Geahlen lab, we have identified a novel cancer target and are designing novel inhibitors directed at that target. Second, in collaboration with the Gibbs lab we have developed a novel series of prodrugs (2) designed to inhibit farnesyl transferase, an important enzymatic target in tumor cells. Although these prodrugs have minimal activity as single agents, in combination with one of the widely used statin drugs they are nanomolar inhibitors of tumor cell proliferation. Finally, we are using the phosphoramidate prodrug technology to develop a novel class of drug-dendrimer nanoparticles that will facilitate targeted delivery of cancer drugs.
Kelley, M. R., Luo, M., Reed, A., Su, D., Delaplane, S., Borch, R., Nyland II, R. L., Gross, M., and Georgiadis, M. Functional analysis of novel analogs of E3330 that block the redox signaling activity of the multifunctional AP endonuclease/redox signaling enzyme APE1/Ref-1. Antioxid. Redox Signal. 2010, 12, (Epub ahead of print).
Nyland II, R. L., Luo, M., Kelley, M. R. and Borch, R. F. Design and synthesis of novel quinone inhibitors targeted to the redox function of apurinic/ apyrimidinic endonuclease 1/redox enhancing factor-1 (Ape1/Ref-1). J. Med. Chem. 2010, 53, 1200-1210. PMCID2834202
Sane, K. M., Mynderse, M., Lalonde, D. T., Dean, I. S., Wojtkowiak,J. W., Fouad, F., Borch, R. F., Reiners, Jr., J. J., Gibbs, R. A. and Mattingly, R. R. A novel geranylgeranyl transferase inhibitor in combination with lovastatin inhibits proliferation and induces autophagy in STS-26T MPNST cells. J. Pharmacol. Exp. Ther. 2010, 333, 23-33. PMCID2846025
Luo, M., Delaplane, S., Jiang, A., Reed, A., He, Y., Fishel, M., Nyland II, R.L., Borch, R.F., Qiao, X., Georgiadis, M. M. and Kelley, M. R. Role of the multifunctional DNA repair and redox signaling protein Ape1/Ref-1 in cancer and endothelial cells: Small molecule inhibition of Ape1’s redox function. Antioxid. Redox Signal. 2008, 10, 1853-1867. PMCID2587278
Wojtkowiak, J. W., Fouad, F., LaLonde, D. T., Kleinman, M. D., Gibbs, R. A., Reiners, J. J. Jr., Borch, R. F. and Mattingly, R. R. Induction of apoptosis in Neurofibromatosis Type 1 malignant peripheral nerve sheath tumor cell lines by a combination of novel farnesyl transferase inhibitors and lovastatin. J. Pharmacol. Exp. Ther. 2008, 326, 1-11. PMID18367665
Wu, W., Sigmond, J., Peters, G. J., and Borch, R. F. Synthesis and biological activity of a gemcitabine phosphoramidate prodrug. J. Med. Chem. 2007, 50, 3743-3746. PMCID2518329
Clark, M. K., Reigard, S. A., Wojtkowiak, J., Chirco, R., Mathieu, P., Reiners, J. J. Jr., Mattingly, R. R., Borch, R. F., and Gibbs, R. A. Synthesis, biochemical, and cellular evaluation of farnesyl monophosphate prodrugs as farnesyltransferase Inhibitors. J. Med. Chem. 2007, 50, 3274-3282. PMID17555307
Boutselis, I. G.; Yu, X.; Zhang, Z. Y.; Borch, R. F. Synthesis and cell-based activity of a potent and selective protein tyrosine phosphatase 1B inhibitor prodrug. J Med Chem 2007, 50, 856-64. PMID17249650
Choi, J. Y.; Borch, R. F. Highly efficient synthesis of enantiomerically enriched 2-hydroxymethylaziridines by enzymatic desymmetrization. Org Lett 2007, 9, 215-8. PMCID2529399

