
Ph.D. - 1980 - University of Wyoming
Postdoc - 1980-82 - Fred Hutchinson Cancer Research Center, Seattle
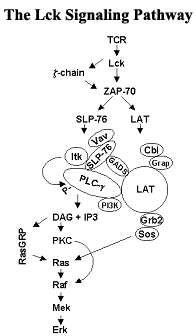
Our group studies the signaling molecule Lck. Lck is a member of the Src-family of protein tyrosine kinases. Lck is required for the activation of T-lymphocytes (T-cells) and individuals lacking functional T-cells fail to develop immune responses. In addition to protecting the body from the invasion of foreign pathogens, the immune system is thought to impact the development of cancer. In the case of cancer vaccine development, the immune system actually impedes the therapy of cancer and harnessing the immune system to aid in the prevention and treatment of tumors has been an ongoing strategy in the war against cancer. The recent discovery that subsets of T cells protect against the development of skin cancer lends powerful support to the original hypothesis that the immune system provides surveillance against developing malignancies. In addition to its requisite role in T cell development and activation, Lck is itself an oncogene and the forced expression of a constitutively active form in mice leads to the development of thymic tumors. Lck is thus an important player in the field of cancer immunology and a critical player in the overall immune response.
We study all aspects of the regulation and function of Lck and the signaling pathways its activation initiates. Recently, we have concentrated on its cellular localization and its modification by serine phosphorylation, which occurs following the binding of antigen to the T-lymphocyte receptor (TCR) as well as during mitosis. Lck is localized to the inner surface of the plasma membrane and is distributed equally between special domains within the plasma membrane (membrane lipid rafts) and the non-raft regions of the membrane. We are interested in determining both the mechanism through which Lck is differentially localized as well as whether the intramembrane localization of Lck influences its ability to initiate signaling through the TCR. Our group additionally is involved in collaborative projects with both the Gibbs and Post laboratories. With the Gibbs lab, we focus on designing and synthesizing chemical analogs of palmitic acid that will prevent Lck from entering lipid rafts. With the Post lab, we focus on determining the three dimensional structure of serine phosphorylated Lck.
Li, M., Ong, S., Bartek, R., Thieu, V.T., Geahlen, R.L., and Harrison, M.L. (2008) "The SH3 Domain of Lck Modulates TCR-dependent Activation of Erk through Activation of Raf-1" Mol. Cell. Biol. 28, 630-641..
Oh, H., Ozkirimli E., Shah, K., Harrison, M.L., and Geahlen, R.L. (2007) "Generation of an analog-sensitive Syk tyrosine kinase for the study of signaling dynamics from the B cell antigen receptor" J. Biol. Chem. 282, 33760-33768.
Krzysiak, A.J.,Rawat, D.S., Scott, S.A., Zhan, T.J., Haworth, K.B., Pais, J.E., Handley, M., Harrison, M.L., Fierke, C.A., and Gibbs, R.A. (2007) "Combinatorial Modulation of Protein Prenylation" ACS Chem. Biol. 2, 385-389.
Zhou, F., Hu, J., Ma, H., Harrison, M.L., and Geahlen, R.L. (2006) "Nucleocytoplasmic trafficking of the Syk protein-tyrosine kinase" Mol. Cell. Biol. 26, 3478-3491.
Zheng, Y.N., Balakrishnan, V., Buzzard, G., Geahlen, R., Harrison, M.L., and Rundell, A. (2005) "Modeling and analysis of early events in T-lymphocyte antigen-activated intracellular-signaling pathways" J. Com. and Applied Math. 184, 320-341.

